Day-2 and day-3 sequential transfer improves pregnancy rate in patients with repeated IVF-embryo transfer failure: a retrospective case-control studyReproductive BioMedicine Online27 January 2013 |

Dr Cong Fang is the associate director of the Reproductive Medicine Research Center of the Sixth Affiliated Hospital of Sun Yat-sen University as well as the director of the IVF–embryo transfer laboratory. She has been working on reproductive medicine as well as relevant research and education projects since 1998, from which she has accomplished rich experience in clinical management of infertility and relevant laboratory procedures. She was the first in China to convene researches on preimplantation genetic diagnosis for patients with chromosome translocations and to report the first successful pregnancy thereby.
PII: S1472-6483(12)00600-1
doi:10.1016/j.rbmo.2012.10.004
© 2012 Reproductive Healthcare Ltd. Published by Elsevier Inc All rights reserved.
The purpose of this study was to evaluate the effect of sequential embryo transfer in patients with repeated IVF failure. A retrospective matched case–control study was conducted and the outcomes of 213 patients with a history of repeated IVF–embryo transfer failure were analysed, of which 33 women underwent sequential embryo transfer on day 2 and day 3 (D2/D3 group), 66 women on day 3 and day 5 (D3/D5 group), 85 women underwent day-3 embryo transfer only (D3 control group) and 29 women underwent day-5 embryo transfer only (D5 control group) in the assisted reproduction centre of the Sixth Affiliated Hospital of Sun Yat-sen University from August 2010 to December 2011. The results showed that the clinical pregnancy rate of the D2/D3 group was higher than that of the D3 group (48.5% versus 22.4%, P![]() =
=![]() 0.006) while the clinical pregnancy rates of the D3/D5 and D5 groups were not significantly different (50.9% versus 45.8%). Day-2 and day-3 sequential embryo transfer may improve the clinical outcomes for patients with repeated IVF–embryo transfer failures.
0.006) while the clinical pregnancy rates of the D3/D5 and D5 groups were not significantly different (50.9% versus 45.8%). Day-2 and day-3 sequential embryo transfer may improve the clinical outcomes for patients with repeated IVF–embryo transfer failures.
Keywords: embryo transfer, implantation rate, IVF, repeated failure
The clinical pregnancy rate following IVF–embryo transfer is usually 40–50% and can be as high as 60% in patients who are treated with IVF–embryo transfer for the first time (Margalioth et al., 2006). However, some patients experience repeated IVF–embryo transfer failures, and the success rate of subsequent IVF cycles in such patients is lower than the overall success rate. The possible reasons for failure include reduced endometrial receptivity, embryonic defects, immune factors, or multifactorial reasons (Lédée-Bataille et al., 2002). Improving the clinical pregnancy rate in these patients is a challenge faced by clinicians practising reproductive medicine and inadequate uterine receptivity is partly responsible for implantation failures. Thus, improving endometrial receptivity is essential to increase the IVF–embryo transfer success rate. In murine experiments, this study group has shown that embryos can induce better endometrial receptivity (Li et al., 2012).
In humans, sequential embryo transfer may be used to increase endometrial receptivity. Machtinger et al. (2006) reported that sequential transfer improves the pregnancy rate in patients with repeated IVF–embryo transfer failures. From 2010, sequential embryo transfer has been utilized for patients with repeated IVF failures in this study centre, and here are analysed the clinical outcomes of sequential and once-only embryo transfer to evaluate the effect of sequential embryo transfer.
Patients undergoing IVF–embryo transfer at the Sixth Affiliated Hospital of Sun Yat-sen University from August 2010 to December 2011 with three or more cycle failures were selected for the study. The inclusion criteria were age ⩽40 years, normal karyotype, normal immunological and thrombophilia screening, absence of endometrial abnormalities by hysteroscopy investigation and polycystic ovary syndrome, endometriosis, availability of  3 good-quality embryos on day 2 and
3 good-quality embryos on day 2 and  2 good-quality embryos on day 3.
2 good-quality embryos on day 3.
During the study period, 165 women in the IVF unit underwent sequential transfer on day 2 and day 3 (D2/D3 group) or day 3 and day 5 (D3/D5 group), of which 33 met the necessary criteria for inclusion in the D2/D3 group and 66 met the inclusion criteria of the D3/D5 group. During the same period, 1310 women received day-3 transfer only, of which 85 women matched with the D2/D3 group in age, basal FSH concentration, cause of infertility, number of previous IVF cycles, number of oocytes retrieved and number of good-quality embryos on day 2 and were recruited as the D3 control group. Of the 115 women who had undergone day-5 transfer only during the same period, 29 cases matched with the D3/D5 group and met the inclusion criteria and so were recruited as the D5 control group.
This study was performed with patients’ consent and was approved by the ethical committee of the Sixth Affiliated hospital of Sun Yat-sen University (reference no. 2010013, approved 29 July 2010).
The standard gonadotrophin-releasing hormone agonist long protocol (mid-luteal phase) was utilized. Briefly, 1.3![]() mg triptorelin depot or 0.1
mg triptorelin depot or 0.1![]() mg triptorelin (IPSEN Pharma Biotech, France) was administered for down-regulation, and 100–300
mg triptorelin (IPSEN Pharma Biotech, France) was administered for down-regulation, and 100–300![]() IU recombinant FSH (Puregon; Organon, Oss, Netherlands; or Gonal-F; Serono, Switzerland) was administered daily for ovarian stimulation. Follicle growth monitoring included serum oestradiol, progesterone and LH measurements and vaginal ultrasound investigation. When one follicle reached a diameter of
IU recombinant FSH (Puregon; Organon, Oss, Netherlands; or Gonal-F; Serono, Switzerland) was administered daily for ovarian stimulation. Follicle growth monitoring included serum oestradiol, progesterone and LH measurements and vaginal ultrasound investigation. When one follicle reached a diameter of  18
18![]() mm or two follicles reached
mm or two follicles reached  17
17![]() mm, 10,000
mm, 10,000![]() IU of human chorionic gonadotrophin (Lizhu Pharmacy, Zhuhai, China) was administered and oocytes were retrieved 36
IU of human chorionic gonadotrophin (Lizhu Pharmacy, Zhuhai, China) was administered and oocytes were retrieved 36![]() h later. Routine IVF or intracytoplasmic sperm injection was performed 4
h later. Routine IVF or intracytoplasmic sperm injection was performed 4![]() h after oocyte retrieval, and the oocytes were checked for fertilization 16–18
h after oocyte retrieval, and the oocytes were checked for fertilization 16–18![]() h later. Normal fertilization was indicated by the appearance of two pronuclei. Embryos were cultured in commercial sequential IVF medium (Quinn‘s Advantage Cleavage Medium; SAGE, Pasadena, CA, USA) for days 2 and 3.
h later. Normal fertilization was indicated by the appearance of two pronuclei. Embryos were cultured in commercial sequential IVF medium (Quinn‘s Advantage Cleavage Medium; SAGE, Pasadena, CA, USA) for days 2 and 3.
Embryos were observed at 48![]() h (day 2) and 72
h (day 2) and 72![]() h (day 3) after oocyte retrieval. The grading criteria for the embryos were as follows: grade 1, the size of the blastomeres was uniform, with no DNA fragmentation; grade 2, the blastomere size was slightly uneven with <20% DNA fragmentation; grade 3, the blastomere size was heterogeneous, or with 20–50% DNA fragmentation; and grade 4, >50% DNA fragmentation. The number and grade of the embryonic blastomeres were recorded. Good-quality embryos were defined as embryos containing
h (day 3) after oocyte retrieval. The grading criteria for the embryos were as follows: grade 1, the size of the blastomeres was uniform, with no DNA fragmentation; grade 2, the blastomere size was slightly uneven with <20% DNA fragmentation; grade 3, the blastomere size was heterogeneous, or with 20–50% DNA fragmentation; and grade 4, >50% DNA fragmentation. The number and grade of the embryonic blastomeres were recorded. Good-quality embryos were defined as embryos containing  4 cells on day 2 (48
4 cells on day 2 (48![]() h after oocyte retrieval) and
h after oocyte retrieval) and  6 cells on day 3 (72
6 cells on day 3 (72![]() h after oocyte retrieval) with a grade of 1–2.
h after oocyte retrieval) with a grade of 1–2.
In the D3 group, embryo transfer was carried out on day 3 after oocyte retrieval. Embryos with normal fertilization and graded as good-quality embryos on day 3 were selected for transfer. No more than three embryos were transferred. In the sequential D2/D3 group, one embryo was transferred on day 2, then remaining embryos were cultured to day 3 and one or two good-quality embryos were transferred. In the sequential D3/D5 group, one good-quality embryo was transferred on day 3, then the remaining good-quality embryos were placed in blastocyst culture medium (Quinn’s Advantage Blastocyst Medium; SAGE) and cultured until day 5. On day 5, one or two blastocysts were transferred. In the D5 group, all of the good-quality embryos were cultured to day 5, and one, two or three blastocysts were transferred. Luteal support was given with 20–40![]() mg progesterone in oil (Xianju, Zhejiang, China) until 14 days after embryo transfer, and progesterone was maintained until 9–12
mg progesterone in oil (Xianju, Zhejiang, China) until 14 days after embryo transfer, and progesterone was maintained until 9–12![]() weeks of gestation in pregnant patients.
weeks of gestation in pregnant patients.
The primary outcome measures were the clinical pregnancy rate and implantation rate. The secondary outcome measure was the miscarriage rate. Pregnancy testing was performed 14![]() days after embryo transfer. Ultrasound examination was performed at week 7 to assess the fetal sac number and the fetal heartbeat. Clinical pregnancy was defined as the presence of a fetal heart beat on ultrasound examination at 7
days after embryo transfer. Ultrasound examination was performed at week 7 to assess the fetal sac number and the fetal heartbeat. Clinical pregnancy was defined as the presence of a fetal heart beat on ultrasound examination at 7![]() weeks of pregnancy. The implantation rate was defined as the number of gestational sacs seen on the ultrasound divided by the total number of embryos/blastocysts transferred. Spontaneous miscarriage was defined as a clinical pregnancy loss before 28
weeks of pregnancy. The implantation rate was defined as the number of gestational sacs seen on the ultrasound divided by the total number of embryos/blastocysts transferred. Spontaneous miscarriage was defined as a clinical pregnancy loss before 28![]() weeks of gestation age (Chinese Medical Association of Obstetrics and Gynecology Society, 2007). Multiple pregnancy was defined as two or more gestational sacs observed on ultrasound.
weeks of gestation age (Chinese Medical Association of Obstetrics and Gynecology Society, 2007). Multiple pregnancy was defined as two or more gestational sacs observed on ultrasound.
The Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) was applied for data analysis. Data were expressed as mean![]() ±
±![]() SD unless stated otherwise. Chi-squared test was used to analyse categorical variables while Student‘s t-test was used for continuous variables. A P-value <0.05 was considered statistically significant.
SD unless stated otherwise. Chi-squared test was used to analyse categorical variables while Student‘s t-test was used for continuous variables. A P-value <0.05 was considered statistically significant.
The indication for IVF, average age and duration of infertility in the sequential transfer and control groups were not significantly different (Table 1). No statistically significant differences existed between the groups with respect to the number of previous failed cycles, number of oocytes retrieved, fertilization rate, cleavage rate and percentage of good-quality embryos.
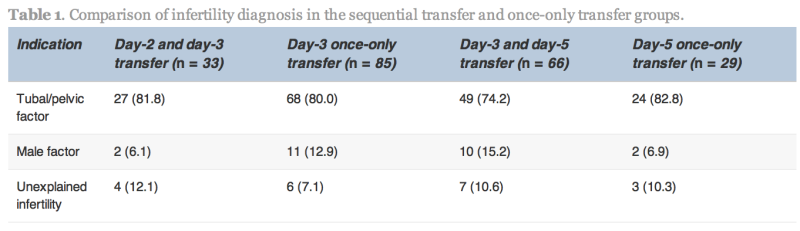
Values are n (%).
No statistically significant differences were found.
The clinical pregnancy and implantation rates in the D2/D3 group were significantly higher than the D3 group (48.5% versus 22.4%, P![]() =
=![]() 0.006 and 18.7% versus 9.3%, P
0.006 and 18.7% versus 9.3%, P![]() =
=![]() 0.018, respectively; Table 2). The multiple pregnancy rate was not significantly different between the two groups.
0.018, respectively; Table 2). The multiple pregnancy rate was not significantly different between the two groups.
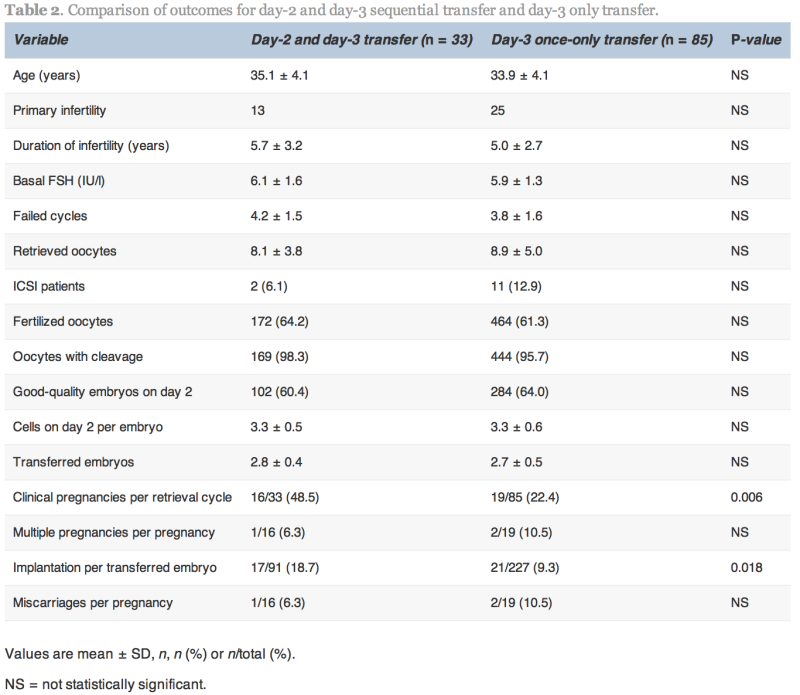
In the D3/D5 group, no blastocysts formed on day 5 in nine cases. In these nine cases, one patient conceived. In the 57 cases with both day-3 and day-5 transfer, 29 (50.9%) achieved pregnancy. In the D5 group, embryo transfer was cancelled because no blastocysts formed on day 5 in five cases, and in the 24 cases with blastocyst transfer, 11 (45.8%) achieved pregnancy.
No statistically significant differences existed between the D3/D5 group and the D5 group with respect to pregnancy and implantation rates; however, the cancellation rate was higher in the D5 group. Higher clinical pregnancy and implantation rates were obtained in the D3/D5 group than the D3 group (43.9% versus 22.4%, P![]() =
=![]() 0.004 and 23.1% versus 9.3%, P < 0.001, respectively; Table 3).
0.004 and 23.1% versus 9.3%, P < 0.001, respectively; Table 3).
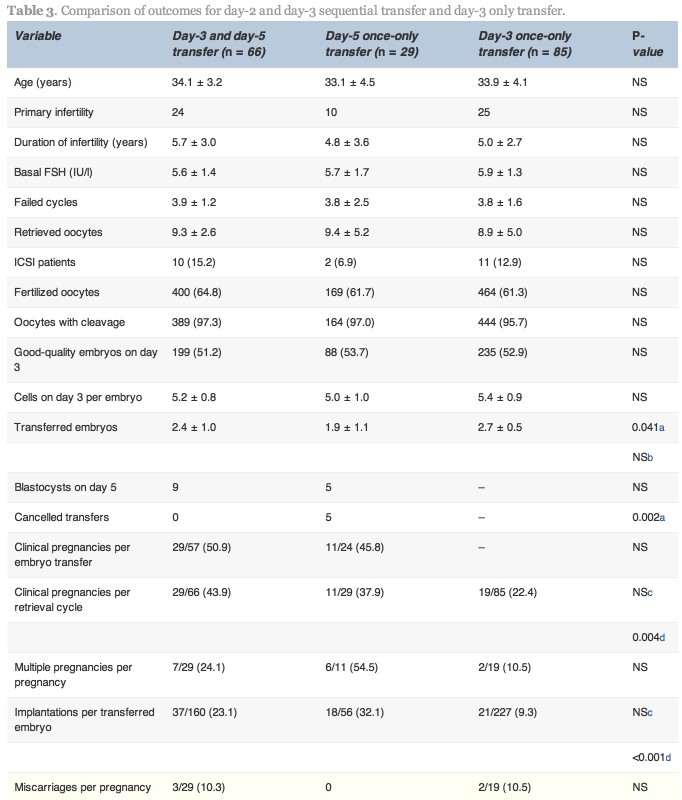
Values are mean![]() ±
±![]() SD, n, n (%) or n/total (%).
SD, n, n (%) or n/total (%).
NS![]() =
=![]() not statistically significant.
not statistically significant.
a,cComparison of the D3/D5 and D5 groups.
b,dComparison of the D3/D5 and D3 groups.
Repeated IVF–embryo transfer failures may occur for a variety of reasons. The typical causes for repeated IVF–embryo transfer failures include reduced endometrial receptivity secondary to uterine cavity anomalies, an excessively thin endometrium, abnormal changes in the expression of adhesion molecules and embryonic developmental abnormalities, such as decreased embryo quality due to a poor culture environment and genetic factors. Some researchers have reported that two-thirds of IVF–embryo transfer failures are due to a lack of endometrial receptivity and one-third due to poor embryo quality (Achache and Revel, 2006). Therefore, at a state-of-the-art reproductive centre, assuming that the embryo culture environment is good, the key to improving the pregnancy rate is to improve endometrial receptivity.
This study’s findings suggest that day-2 and day-3 sequential transfer or day-3 and day-5 sequential transfer can improve the clinical pregnancy and implantation rates in patients with repeated IVF–embryo transfer failures. In the case of day-2 and day-3 sequential transfer, one embryo was transferred on day 2, which may induce an increase in endometrial receptivity, thereby creating a better endometrial environment for the second transfer on day 3. Studies have shown that co-culture of early-stage embryos with endometrial epithelium may increase the success rate of IVF–embryo transfer, especially in patients with repeated failures, indicating that the interaction between the embryo and the endometrium is important (Mercader et al., 2003,Tan et al., 2005, Spandorfer et al., 2006, Eyheremendy et al., 2010). At the same time, it has been shown that embryos can induce an increase in endometrial receptivity (Wakuda et al., 1999). Therefore, during sequential transfer, the embryo transferred on day 2 is co-cultured with the endometrium, which may improve the embryonic development potential and induce an increase in endometrial receptivity, thereby facilitating implantation of the sequentially transferred embryo. Also, mechanical stimulation of the endometrium has been reported to increase the pregnancy rate in patients with repeated IVF–embryo transfer failures (Barash et al., 2003, Zhou et al., 2008). During the first transfer on day 2, insertion of the catheter may be some kind of mechanical stimulation of the endometrium, inducing an increase in endometrial receptivity. Finally, increasing the likelihood of transferring embryos at the receptivity window of the endometrium by sequential transfer has been cited by some authors as another explanation for improved success rates in patients with repeated IVF/embryo transfer failures (Loutradis et al., 2004, Almog et al., 2008).
Transfer on day-3 and day-5 also improved the clinical pregnancy and implantation rates in patients with repeated IVF–embryo transfer failures compared with day-3 transfer, but no significant difference existed between the D3/D5 and D5 groups. It is well known that blastocyst transfer increases the likelihood for synchronized endometrial and embryonic development and endometrial receptivity, thus increasing the implantation rate. In two large-sample, controlled, prospective studies (Guerif et al., 2004, Levitas et al., 2004), blastocyst transfer was shown to significantly improve the implantation and live birth rates in patients with repeated IVF–embryo transfer failures; however, it is possible that no blastocyst forms during blastocyst culture so that no embryo can be transferred. Day-3 and day-5 transfer ensures transfer, on day 3, thereby reducing the effect of the high risk of cancellation of blastocyst transfer. In this study, the clinical pregnancy and implantation rates in the D3/D5 group were not superior to those in the D5 group, indicating that endometrial receptivity on day 5 is sufficient. Thus, the putative effects of the first transferred embryo and the mechanical stimulation by the first transfer have not been observed. However, it may be that statistical significance was not reached due to small sample sizes.
Concern remains regarding the risk of multiple pregnancy associated with sequential embryo transfer due to the high number of embryos transferred. In this study, the number of transferred embryos was similar between the D2/D3 and D3 groups; no difference existed in the incidence of multiple pregnancies. The D3/D5 group had a higher number of transferred embryos than the D5 group, but the incidence of multiple pregnancies was not different.
Ashkenazi et al. (2000) suggested that the second transfer procedure might have a deleterious influence, possibly related to infection or trauma, on the implantation of embryos transferred earlier. However, Tur-Kaspa et al. (1998) reported no significant differences in pregnancy rates with and without immediately repeated transfers. The current study concurs withTur-Kaspa et al. (1998) and shows that the second transfer had no adverse effect on the implantation process.
In addition, some researchers have reported that sequential transfer also improved the pregnancy rate of patients undergoing IVF–embryo transfer for the first time, and the beneficial effects of sequential embryo transfer on the implantation rate were also confirmed (Goto et al., 2003). Therefore, this method may be used not only in patients with repeated failures but also in poor-prognosis patients even in their first IVF–embryo transfer cycle in order to improve the pregnancy rate.
In conclusion, for patients with repeated IVF–embryo transfer failures, sequential transfer on day 2 and day 3 or on day 3 and day 5 may improve the clinical pregnancy rate in cases where no less than two good-quality embryos are available.
This study was supported by the National Natural Science Foundation of China (Grant no. 81070495) and the Natural Science Foundation of Guangdong Province (Grant no. 9151008901000018).
http://download.journals.elsevierhealth.com/pdfs/journals/1472-6483/PIIS1472648312006001.pdf

 (6)
(6)
16812
 sequential ET - multiple pregnancy
sequential ET - multiple pregnancy